When the goal is overall survival, give your AYA patients their best chance* with an asparaginase-containing regimen1,2
Treating AYA patients with asparaginase-containing regimens has shown improved overall survival3
In CALGB 10403, a prospective study, US cancer cooperative groups evaluated overall and event-free survival in AYA patients with ALL treated with a full pediatric regimen.3
*Compared with patient outcomes with hyper-CVAD.
Estimated 3-year overall survival in CALGB patients aged 16-39 years3
Asparaginase-intensive
CALGB 10403
(patients aged 17-39 years)
0 %
Adult
regimen
(patients aged 16-29 years)
0 %
Regimens containing asparaginase provided ~6.5 years of median event-free survival in AYA patients3
In CALGB 10403, median event-free survival in AYA patients was 78.1 months (95% CI: 41.8-NE months) compared to 30 months with historical controls† (95% CI: 22-38 months)3
†Historical controls consisted of patients with newly diagnosed, non-PH+ ALL age 16 to 29 who were enrolled in previous CALGB trials.3
3-year event-free survival for ALL patients treated with pediatric CALGB 10403 regimen containing asparaginase3
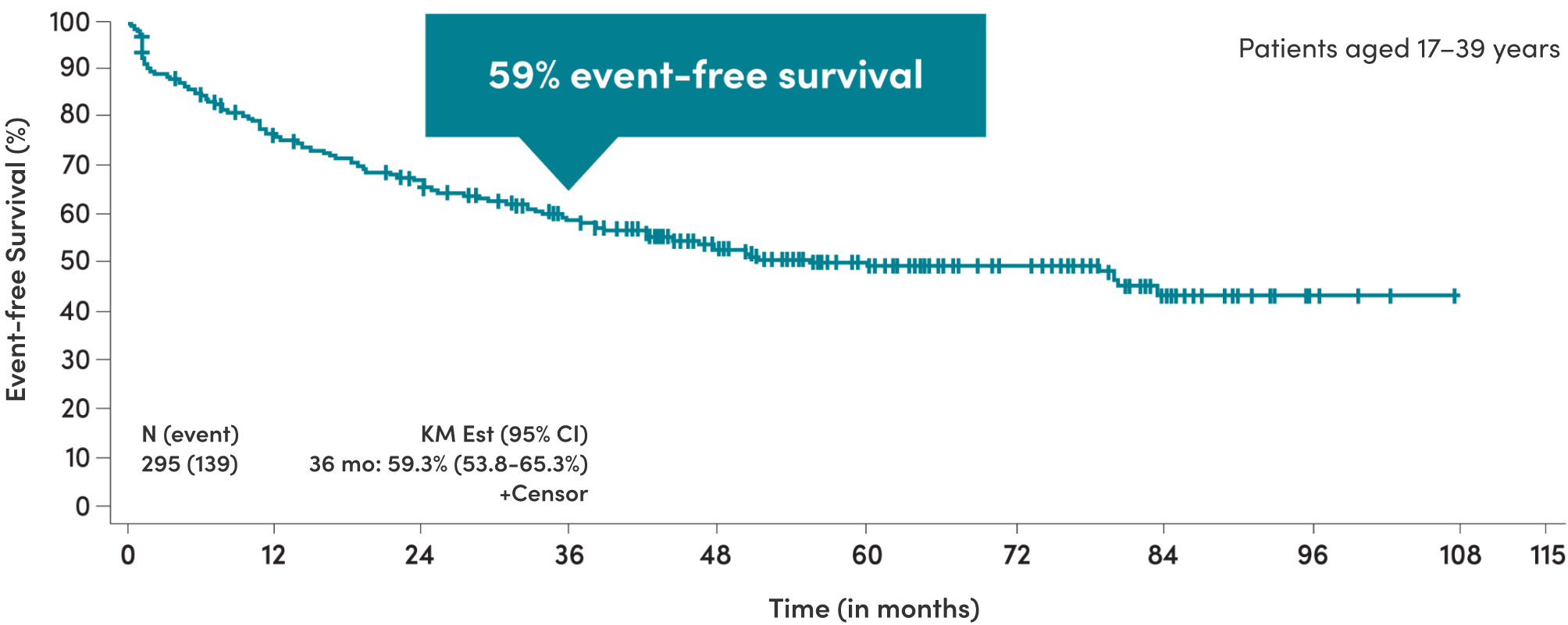
Adapted from Stock et al, 2019.3
The Stock study determined that the CALGB 10403 regimen containing asparaginase was considered safe. Of the 295 evaluable AYA patients, there were 8 (3%) treatment-related deaths: 6 occurred during the induction course and 2 post-remission.3
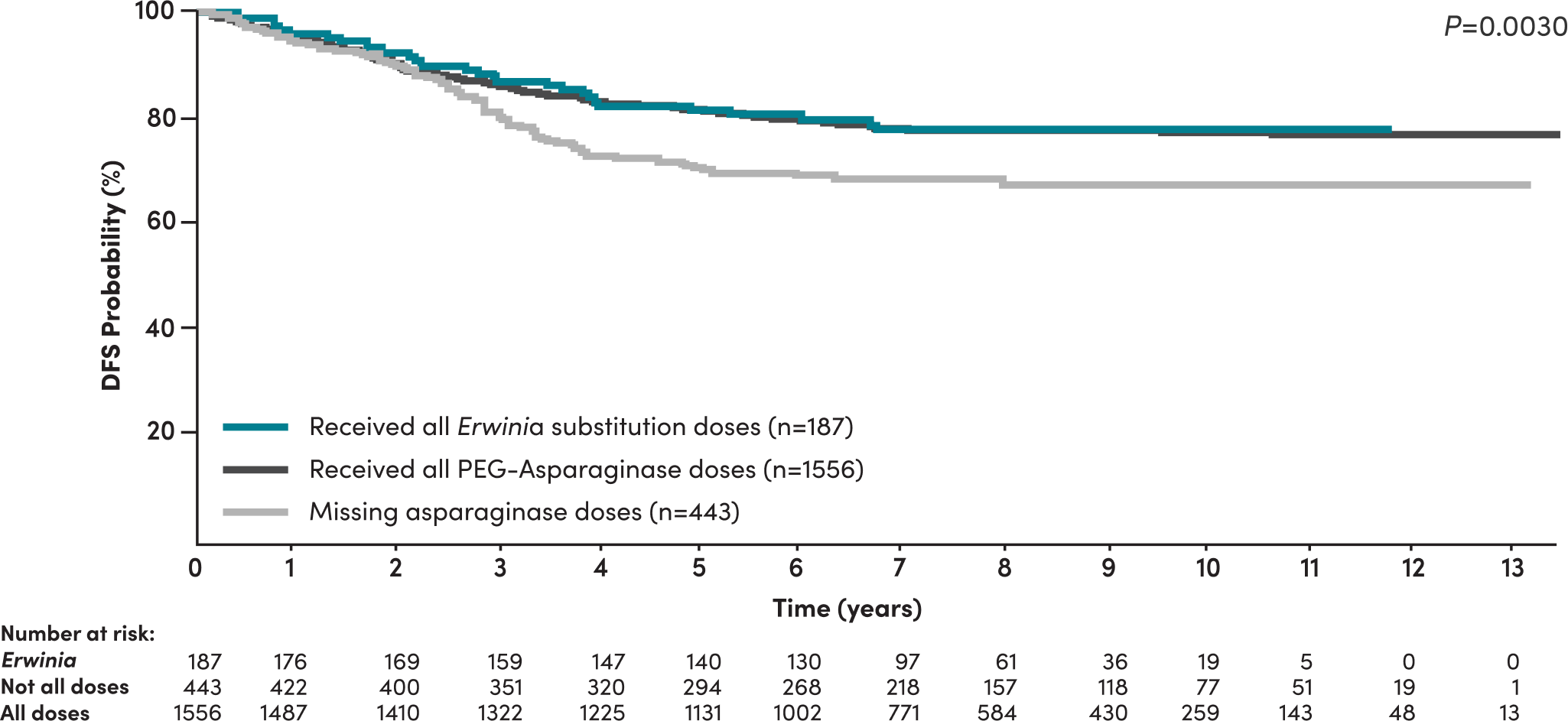
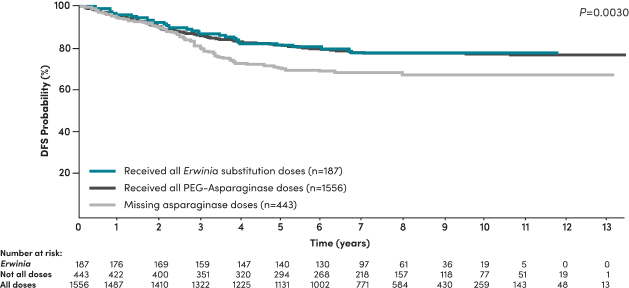
Patients who missed asparaginase doses had inferior disease-free survival (DFS)4
A 2020 study by Gupta et al in high-risk B-ALL COG patients demonstrated4:
- Significantly inferior DFS was seen in patients who did not receive all prescribed asparaginase doses
- Asparaginase missed doses is a predictor of DFS among patients, even when adjusted for other risk factors such as age, cytogenetics, time to response, WBC count, and CNS status
- Missing asparaginase doses increased the risk of an event‡ by 50% (HR=1.5, 95% CI: [1.1-1.9], P=0.002)§
DFS of NCI high-risk patients stratified by asparaginase received4
Commit to asparaginase completion
Patients receiving all Erwinia substitution doses achieved similar DFS compared to patients receiving all pegaspargase doses4
‡Event defined as DFS from time of maintenance initiation to relapse, death, development of a second malignant neoplasm, or last follow-up.4
§Patients with NCI high-risk B-ALL.4
A comprehensive 8-year (2004-2011) study including over 8300 patients, in partnership with the COG.4
The study included newly diagnosed B-ALL patients aged 1-31 years who were enrolled in 1 of 2 COG clinical trials. This included 5195 patients within the COG study AALL0331 (NCI SR B-ALL, age >1 and <10 years, initial WBC count <50,000/μL; 2005-2010) and 3001 patients within AALL0232 (NCI high-risk B-ALL, aged 10-30 years or initial WBC count ≥50,000/μL and any age; 2004-2011, or NCI SR with testicular disease or some patients with steroid pretreatment).4
This landmark survival analysis was for the 2,186 NCI high-risk patients who started maintenance. DFS was defined as time from maintenance initiation to relapse, death, development of a second malignant neoplasm, or last follow-up.4
In high-risk patients who did not receive all prescribed asparaginase doses (n=443), 99 (22.3%) had an event, including 95 (21.3%) with relapse, 2 (0.4%) with second malignant neoplasms, and 2 (0.4%) who died off therapy as a first event.4
There were several study limitations, including (but not limited to)4:
- Exact number of missed pegaspargase doses could not be determined
- Generalizability of our results to patients with T-ALL is unknown
Switching to an asparaginase with minimal immunologic cross-reactivity can help preserve patient outcomes5
- Compared to those who experienced hypersensitivity and subsequently missed doses, higher risk (high-risk + slow early responders for SR) patients who switched to an Erwinia-derived asparaginase therapy due to hypersensitivity were at lower risk of relapse and had higher rates of DFS4
- Rechallenging patients can compromise their outcomes and further expose them to hypersensitivity6,7
When hypersensitivity threatens patient outcomes, consider switching to an immunologically distinct asparaginase8
Help protect patient outcomes
RYLAZE is the only FDA-approved Erwinia asparaginase for the treatment of ALL/LBL for patients who develop hypersensitivity to E. coli asparaginase9
